Subscribe to our Telegram channel to get a daily dose of business and lifestyle news from NHA – News Hub Asia!
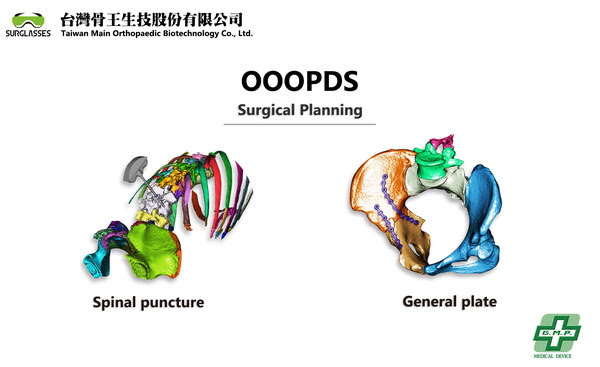
In recent years, 3D printing technology has gradually matured and has been applied in various related medical fields such as orthopedics, spine, dentistry, etc. However, the certified 3D medical imaging surgical planning is the key point that the printed 3D materials transfer into actual medical products. There is very few software like OOOPDS that offered easy-to-use functions the support not only the ultrafast conversion of DICOM into accurate 3D objects but also the one-touch segmentation for the surgical planning to simulate the surgical outcome. It is compatible to connect with 3D printers to print medical implants including metallic materials.
Patient-specific implants using 3D printers have proved their advantages in many research papers in recent decades. Using the patient’s CT and MRI scans to convert into doctors’ surgical plans and through 3D printing to generate custom implants is the best solution for implants. The main part is a key certificated medical software for the applications. OOOPDS is now ready to go into the market with TFDA in Taiwan. For more information, please refer to our official website on www.surglasses.com.
The CEO Wang Min-Liang said Taiwan Main Orthopaedic Biotechnology is well-known innovative medical device company that developed the world’s first smart surgical glasses with Augmented Reality technology. Our smart surgical glasses products obtained the CE mark, and the product has been deployed in the UK, Europe, the Middle East, India, Russia, and other regions. The company is very innovative when it comes to medical software and algorithms. The OOOPDS software is one of our key projects while the company startup. We are very happy to announce the TFDA certification for OOOPDS and we welcome any kind of cooperation.
