- Total planned investment is around 500 million USD over five years to raise manufacturing capacity
- Continues long-term strategy of increasing capacity to respond to growing industry demand and new market opportunities
- Cytiva hiring nearly 1,000 personnel in Austria, China, Singapore, Sweden, Switzerland, and the United States
- New manufacturing lines, 24/7 shift patterns, and increased automation will deliver additional manufacturing capacity
Based on the press release issued by Cytiva, a global life sciences leader, is expanding its manufacturing capacity and hiring personnel in key areas to support the long-term growth of the biotechnology industry.
Emmanuel Ligner, President and CEO, Cytiva, says: “We know from our customers that availability and lead time are the most important considerations after quality. Cytiva’s long-term commitment is to deliver the best product, at the right time, and support our customers with expertise. The industry is growing rapidly, now even more due to COVID-19. Accelerating this investment will reinforce these commitments.”
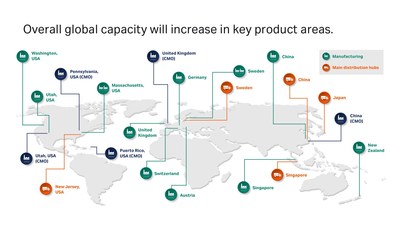
While the COVID-19 pandemic is increasing short-term demand, the biotherapeutics industry was already predicted to grow by double digits between now and 20251. Cytiva’s global product manufacturing and distribution network encompasses 13 sites across Asia, the Americas, and Europe. The investments, totaling around 500 million USD, will respond to in-region, for-region demand, bolster security of supply through dual manufacturing, and increase overall global capacity in key product areas.
Cytiva is investing in talent, too, hiring nearly 1,000 people around the world. Ligner says: “We’re acquiring talent in every area of our business, from commercial to those on production lines, in order to better serve customers for the long term.”
Single-use technologies are used to manufacture 85%2 of the biologics currently in pre-commercial and clinical manufacturing lines. As regulatory approvals occur, demand for single-use products at manufacturing scale is expected to grow substantially. Through additional equipment and infrastructure at multiple sites, Cytiva’s capacity to manufacture single-use products will more than double.
In Asia-Pacific, single-use capacity will triple through a partnership with one of the largest healthcare technology suppliers in China, Wego, which is already producing consumables for Cytiva’s customers in the region.
Cell culture media production will increase thanks to new manufacturing lines and cleanroom space in Logan, Utah, as well as additional shifts and personnel. The Singapore and Pasching, Austria locations are increasing output through more personnel and additional work shifts. This follows on from a tenfold increase in powdered cell culture media production announced in May 2018.
The manufacturing capacity of Cytiva’s MabSelect and Capto chromatography product platforms has doubled, as part of a 70 million USD per year (2017 – 2022) capacity gains and facility modernization program at its Uppsala, Sweden site. Now, the plant is fully automated with the latest technology to allow continuous manufacturing. Other elements include the capacity extension of the Sephadex resin, setting-up additional facilities for in-house manufacturing, and the development of automation and digitalization infrastructure.
Cytiva is also enabling the rapidly growing cell and gene therapy market through an investment in a new facility in Grens, Switzerland to manufacture single-use kits for its Sepax and Sefia cell processing systems.
Cytiva has a longstanding and comprehensive Security of Supply program in place which enables manufacturing output to respond to market demands while ensuring that operations and service capabilities continue safely. For some product lines, part of the solution is having multiple sites able to deliver to customers.
Ligner says: “Dual manufacturing assures our customers that if one location encounters capacity constraints, we have plenty of back-up ready to activate.”

Source : Cytiva





